TOKYO--(BUSINESS WIRE)--Kaneka Corporation (Headquarters: Minato-ku, Tokyo; President: Minoru Tanaka)(TOKYO:4118) has acquired all shares of Japan Medical Device Technology Co., Ltd. (Headquarters: Kamimashiki-gun, Kumamoto; President: Shuzo Yamashita) (hereinafter JMDT), a developer and manufacturer of medical devices, on November 30, 2023, and made it a wholly owned subsidiary.
Kaneka manufactures and sells endovascular catheters, which are devices used in the treatment of cardiac, peripheral vascular, and cerebrovascular diseases. In particular, stenting*1 for the treatment of coronary artery diseases such as atherosclerosis has a market worth 30 billion yen in Japan, and Kaneka is aiming to expand its business in this area.
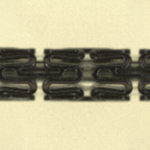
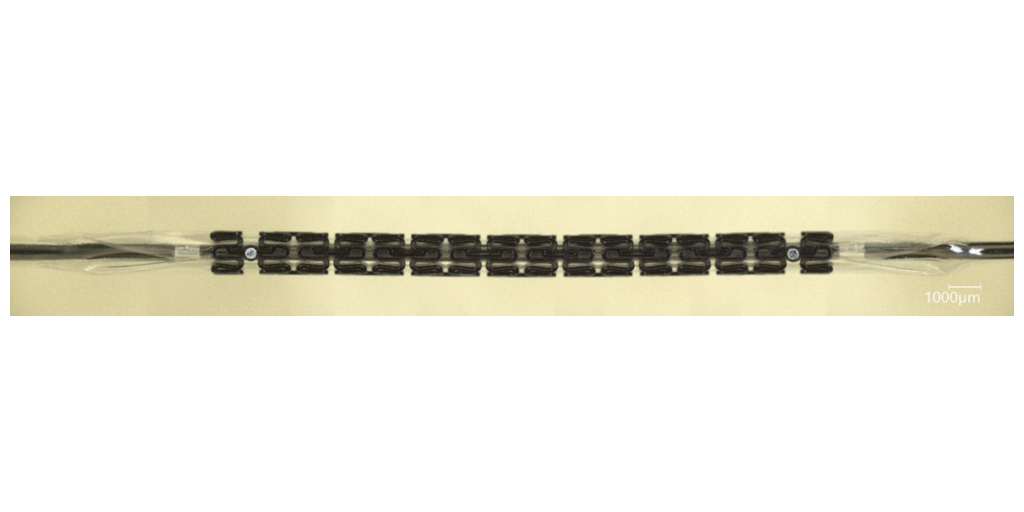
JMDT has high technological capabilities in the research and development of coronary stents and is one of the leaders in the development of bioresorbable stents*2.
Coronary artery treatment usually involves the placement of a tubular metal mesh stent at the lesion to expand the lumen and restore blood flow. However, as the stent remains in the blood vessel, another stent often cannot be placed in the same location should restenosis recur. JMDT's bioresorbable stent is made of a biodegradable magnesium alloy, which prevents vessel blockage potentially caused by the stent remaining in the vessel. In addition, 95% of the stent is resorbed and taken up by the body 1.5 years after implantation, ensuring that it does not interfere with the treatment of restenosis.
*1. A stent is a small, expandable, and tubular metal mesh, often made of metals such as cobalt-chromium alloy or nickel-titanium alloy. It is a medical device that widens tubular parts of the human body (blood vessels, trachea, esophagus, duodenum, colon, biliary tract, etc.) from inside the lumen. They are mounted on a catheter and dilated inside arteries to treat narrowed or occluded coronary arteries or peripheral arteries in the lower extremities. Implanting a stent restores blood flow at the treatment site.
*2. JMDT's bioresorbable stent is made of a highly safe magnesium alloy and has a low risk of thrombosis with a thin (100 µm) yet fully expandable stent body. Its surface is coated with a layer that controls the degradation of the magnesium alloy and drug (Sirolimus) elution. Furthermore, it is currently the only bioresorbable stent in Japan that has progressed to the FIH (First In Human) trial for use in humans for the first time.
|
[Japan Medical Device Technology Co., Ltd.] |
|
|
Representative: |
Shuzo Yamashita |
|
Capital Stock: |
10 million yen |
|
Headquarters: |
Kumamoto Techno Incubation Center Bldg. D, 2020-3 Tabaru, |
|
|
Mashiki-machi, Kamimashiki-gun, Kumamoto, 861-2202 |
|
Foundation: |
October 2014 |
|
Business: |
Research and development for coronary stents and commissioned development of other medical devices |
Introduction video of the bioresorbable stent (by JMDT):
https://www.youtube.com/watch?v=CrFBhuQCIf4
Contacts
KANEKA CORPORATION
Investors & Public Relations Department
Chika Harada
[email protected]