SINGAPORE--(BUSINESS WIRE)--#coronavirus--Molecular diagnostics company Lucence announced today the development of the SAFER-Sample (Stabilization of nucleic Acid Formulation for Evaluation of RNA) kit, a sample collection medical device to facilitate more accessible testing of viral infections such as COVID-19.
Presently, definitive diagnosis of RNA viral infections such as COVID-19 requires patients with respiratory infections to undergo nose and/or throat swabs, and it is recommended that these samples are transported with chilled media. However, chilled transport media is not universally available and subject to worldwide shortages, leading to the risk of compromised testing because of specimen drying, contamination or degradation, especially if transported at room temperature. Among other factors, accurate diagnostic testing depends on sampling quality, storage and transport to the testing laboratory.
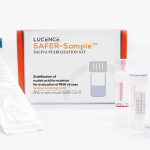
The SAFER-Sample medical device is a collection kit that comes with a bottle of stabilization fluid to be mixed with the sample at the point of collection, keeping viral RNA stable at room temperature for up to one week. The sample can then be transported to a testing lab without the need for chilling, especially useful in countries where samples must be transported across large geographical areas for testing.
Dr. Tan Min-Han, Founding CEO of Lucence and Adjunct Clinician-Scientist at the Institute of Bioengineering and Nanotechnology (IBN) of A*STAR said, “We are very glad that our technology, used to reduce suffering of cancer patients, can contribute to accurate testing solutions during this global crisis. In line with our mission of using molecular technology to improve health worldwide, we are making up to 10,000 kits available at no cost to the scientific community.”
The reagent used in the SAFER-Sample kit was invented at the Institute of Bioengineering and Nanotechnology of A*STAR, the Agency for Science, Technology and Research of Singapore. Experiments demonstrate that SAFER-Sample allows for better stabilization of the genomes of RNA viruses, versus other types of specimen transport media at room temperature, to facilitate more accurate testing.
Additional evaluation of SAFER-Sample’s ability to inactivate COVID-19 at collection is in progress to understand the kit’s ability to improve the safety of healthcare workers while they collect and process patient samples. With Lucence’s expertise in non-invasive ultrasensitive molecular testing technology, and the productizing capabilities of the Diagnostics Development (DxD) Hub, SAFER-Sample kit can also be used for non-invasive sample types including saliva. DxD Hub is a national initiative led by A*ccelerate, the commercialization arm of Singapore’s Agency for Science, Technology and Research.
Dr. Sidney Yee, CEO of DxD Hub said “We’re excited that this collaboration can result in more accessible testing of RNA viruses. Such public-private partnerships are especially important in times like these, where the translation of innovation in the labs to rapidly fulfill the demands of the healthcare community is critical.”
The National Centre for Infectious Diseases (NCID) Singapore is currently evaluating non-invasive patient specimens collected with the SAFER-Sample reagent, and Lucence is working with the Ministry of Health Singapore for broader performance evaluation. The SAFER-Sample kit has been registered with the Health Sciences Authority (HSA) as a Class A medical device.
A/Prof Hsu Li Yang, Program Leader in Infectious Diseases, Saw Swee Hock School of Public Health at the National University of Singapore (NUS), and Adjunct Clinician-Scientist at IBN, said, “This innovation comes at a time when primary care and community testing of COVID-19 is becoming an urgent need. Public-private partnerships that result in these breakthroughs are critical for delivering necessary resources in areas that either are unable to achieve alone.”
About Lucence
Lucence is a precision oncology company founded on a vision of a world without avoidable cancer deaths. The company makes state-of-the-art, highly sensitive liquid biopsy tests for disease detection and treatment selection. Headquartered in Singapore and Palo Alto, California, Lucence supplies molecular diagnostic services for innovative cancer testing through a US federally CLIA-licensed, CAP-accredited central laboratory. For more information, visit www.lucence.com.
Contacts
Gabriel Yap
media@lucence.com


.jpg)








