Celltrion Accelerates Development of COVID-19 Antiviral Treatment and Aims to Launch Rapid Self-testing Kit


- Celltrion completes first step of developing an antiviral treatment to fight COVID-19 and hopes to begin human clinical trials in the third quarter of 2020
- Celltrion aims to launch the rapid self-testing diagnostic kit for COVID-19 this summer: the kit is designed to provide results within 15-20 min of self-testing
- Leveraging its world-class expertise in biologics, Celltrion Group remains committed to supporting patients and healthcare systems throughout the pandemic
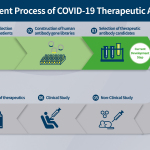
INCHEON, South Korea–(BUSINESS WIRE)–Celltrion Group today announced key milestones in its efforts to fight the 2019 novel coronavirus (COVID-19) pandemic. As part of its emergency preparedness plan to address the outbreak, which has infected over 200,000 people worldwide,1,2 Celltrion has successfully completed the first step of developing an antiviral treatment to fight COVID-19 and aims to launch a rapid self-testing diagnostic kit that could provide results within 15-20 minutes.
Celltrion has been selected as a preferred developer for a monoclonal antibody project to treat and prevent COVID-19 by the Korea Centers for Disease Control (KCDC). Korea was one of the first countries to be affected by the global pandemic.3 Celltrion has identified the library of antibodies sourced from the blood of recovered patients in Korea, which are thought to be involved in neutralising the virus and may contribute to recovery from COVID-19. These antibodies are undergoing further screening processes to identify those that are most effective in neutralising the virus causing COVID-19. Once identified these will form the basis of the antiviral treatment to be tested through pre-clinical and clinical trials around the world in the third quarter of 2020.
Furthermore, Celltrion plans to develop a ‘super antibody’ that can attach and neutralise all kinds of coronavirus related strains, such as those causing COVID-19 and SARS, enabling further protection against unforeseen or unexpected mutations. Celltrion hopes the ‘super antibody’ candidate could contribute towards preparedness for potential future pandemic situations.
Alongside therapeutic antibody development, Celltrion aims to launch a rapid self-testing diagnostic kit in the summer of this year. The kit will focus on the gene that encodes the surface spike (S) protein; an essential glycoprotein for viral entry into human host cells. The kit is designed to show results within 15-20 minutes, with optimised sensitivity, specificity and improved accuracy features. Once it has gained a CE mark, the rapid self-testing diagnostic kit will become available throughout Europe through Celltrion Healthcare. Celltrion plans to apply for device authorisation from the FDA in the US and other regulatory authorities after acquiring relevant data.
Ki-Sung Kwon, Head of R&D Division at Celltrion said, “Our COVID-19 antiviral treatment is designed to train the immune system to make antibodies that recognise and block the spike protein that the virus uses to enter human cells. Antibodies that bond to the COVID-19 antigen will also be used to develop a diagnostic kit. We implemented an emergency preparedness plan at the very start of the COVID-19 outbreak leveraging our unique antibody discovery, development and manufacturing technologies. We remain dedicated to supporting healthcare systems across the world and innovating to support patients throughout this pandemic.”
– ENDS –
Notes to Editors:
About Celltrion Healthcare
Celltrion Healthcare is committed to delivering innovative and affordable medications to promote patients’ access to advanced therapies. Its products are manufactured at state-of-the-art mammalian cell culture facilities, designed and built to comply with the US FDA cGMP and the EU GMP guidelines. Celltrion Healthcare endeavours to offer high-quality cost-effective solutions through an extensive global network that spans more than 110 different countries. For more information please visit: https://www.celltrionhealthcare.com/en-us
About COVID-194
Coronaviruses (CoV) are a family of viruses that lead to illnesses from the common cold to severe diseases such as Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV). Novel coronavirus SARS-CoV-2 is responsible for the disease COVID-19, this new strain, discovered in 2019, is behind the ongoing pandemic outbreak.
Common signs of COVID-19 include respiratory symptoms, fever, cough, shortness of breath and breathing difficulties. In severe cases, infection can lead to pneumonia, severe acute respiratory syndrome, kidney failure and ultimately death.
Please find up to date information about the outbreak via the World Health Organization at https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
FORWARD LOOKING STATEMENT
Certain information set forth in this press release contains statements related to our future business and financial performance and future events or developments involving Celltrion/Celltrion Healthcare that may constitute forward-looking statements, under pertinent securities laws.
These statements may be identified by words such as “prepares”, “hopes to”, “upcoming”, ”plans to”, “aims to”, “to be launched”, “is preparing, “once gained”, “could”, “with the aim of”, “may”, “once identified”, “will”, “working towards”, “is due”, “become available”, “has potential to”, the negative of these words or such other variations thereon or comparable terminology.
In addition, our representatives may make oral forward-looking statements. Such statements are based on the current expectations and certain assumptions of Celltrion/Celltrion Healthcare’s management, of which many are beyond its control.
Forward-looking statements are provided to allow potential investors the opportunity to understand management’s beliefs and opinions in respect of the future so that they may use such beliefs and opinions as one factor in evaluating an investment. These statements are not guarantees of future performance and undue reliance should not be placed on them.
Such forward-looking statements necessarily involve known and unknown risks and uncertainties, which may cause actual performance and financial results in future periods to differ materially from any projections of future performance or result expressed or implied by such forward-looking statements.
Although forward-looking statements contained in this presentation are based upon what management of Celltrion/Celltrion Healthcare believes are reasonable assumptions, there can be no assurance that forward-looking statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Celltrion/Celltrion Healthcare undertakes no obligation to update forward-looking statements if circumstances or management’s estimates or opinions should change except as required by applicable securities laws. The reader is cautioned not to place undue reliance on forward-looking statements.
References
1 Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). John Hopkins University. Available at: https://coronavirus.jhu.edu/map.html Last accessed: March 2020
2 Novel Coronavirus (COVID-19) Situation. World Health Organization. Available at: https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd Last accessed: March 2020
3 Novel Coronavirus – Republic of Korea (ex-China). World Health Organization. Available at: https://www.who.int/csr/don/21-january-2020-novel-coronavirus-republic-of-korea-ex-china/en/ Last accessed: March 2020
4 Coronavirus. World Health Organization. Available at: https://www.who.int/health-topics/coronavirus Last accessed: March 2020
Contacts
Zuzanna Grzeskiewicz
zgrzeskiewicz@hanovercomms.com
+44 (0) 7506 339043
Preetika Ramjoorawon
pramjoorawon@hanovercomms.com
+44 (0) 7595 700045
Recent Posts
AIA Hong Kong continues to lead the insurance industry with 9 market No.1 in 2024
Number of New Business Policies tops the market for 11 consecutive years HONG KONG SAR…
Feng Wei Ju and 8½ Otto e Mezzo BOMBANA Garner Coveted Diamond Awards in Black Pearl Restaurant Guide 2025
MACAU SAR - Media OutReach Newswire - 25 April 2025 - 2025 Black Pearl Restaurant…
Creww and Real Madrid Next launch Batch 2 of “Real Madrid Next Accelerator for Asia”
TOKYO, JAPAN - Media OutReach Newswire - 25 April 2025 – Creww Inc. (Japan Office:…
Digital Storytelling: “Zhengzhou in Cultural Relics” Debuts with AR Reconstructions Global Premiere on International Day for Monuments and Sites
ZHENGZHOU, CHINA - Media OutReach Newswire - 25 April 2025 – On International Day for…
Chinese and foreign guests gather to talk about innovation and development of Museum in Liangzhu, Hangzhou
HANGZHOU, CHINA- Media OutReach Newswire - 25 April 2025 - From April 23 to 25,…
Hang Lung’s 65th Anniversary Celebrations Arrive at Shanghai Grand Gateway 66 and Plaza 66 Debut of “ButterBear” & Takashi Murakami’s Ohana Hatake Pop-up
Fuel double-digit growth in foot traffic and sale HONG KONG SAR and SHANGHAI, CHINA -…