Grant From Monash University’s Artificial Heart Frontiers Program, supported by the Australian Government’s Medical Research Future Fund, to advance medical technologies for severe heart failure
GOLD COAST, Australia & HUNTINGTON BEACH, Calif.–(BUSINESS WIRE)–BiVACOR®, a clinical-stage medical device company, today announced $13 million (USD) awarded from the Australian Government’s Medical Research Future Fund (MRFF) grant through the Artificial Heart Frontiers Program (AHFP) to support BiVACOR’s Total Artificial Heart program and future product enhancements.
Led by Monash University, the AHFP is comprised of a consortium of research centers in Australia in collaboration with BiVACOR. The $13 million (USD) grant comes from a larger $50 million (AUD) grant from the MRFF to the AHFP to develop and commercialize devices to treat the most common forms of severe heart failure and bring new solutions to underserved patients.
The award will support clinical work of the BiVACOR Total Artificial Heart (TAH) and begin development for the integration of wireless power sources for the device.
An FDA-granted investigational device exemption (IDE) first-in-human Early Feasibility Study (EFS) for BiVACOR’s Total Artificial Heart is slated to begin in the first half of 2024, working with two pioneers and luminaries in cardiovascular surgery, William E. Cohn, MD and O.H. (Bud) Frazier, MD, of the Texas Heart Institute.
“There is a huge gap between available treatment options and the number of patients with severe heart failure. Initiating human clinical work for the BiVACOR TAH is the first step to address critical patient needs from this non-curative disease,” said Daniel Timms, Ph.D., BiVACOR Founder and Chief Technology Officer. “We are honored to be a part of the Artificial Heart Frontiers Program, working with our close partners: lead institution Monash University, The University of New South Wales, The University of Queensland, and Griffith University, as well as our clinical partners, St. Vincent’s Hospital, Sydney, and The Alfred Hospital. The Australian government’s investment further validates the dire need for innovation in this field. It is a testament to the promise of our technology to bring these life-saving devices to market over the next few years.”
“Heart failure is a chronic progressive condition in which patients suffer from debilitating symptoms, including persistent breathlessness and fatigue, that frequently require hospitalization at great cost to a patient’s quality of life and the health system,” said project co-lead and Director of Cardiology at The Alfred, Professor David Kaye, who also leads the Monash Alfred Baker Centre for Cardiovascular Research. “The average survival of a heart failure patient is comparable to some cancers at just five years and is even less for patients with advanced heart failure, who are the people our devices will most benefit.”
About the BiVACOR Total Artificial Heart
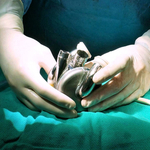
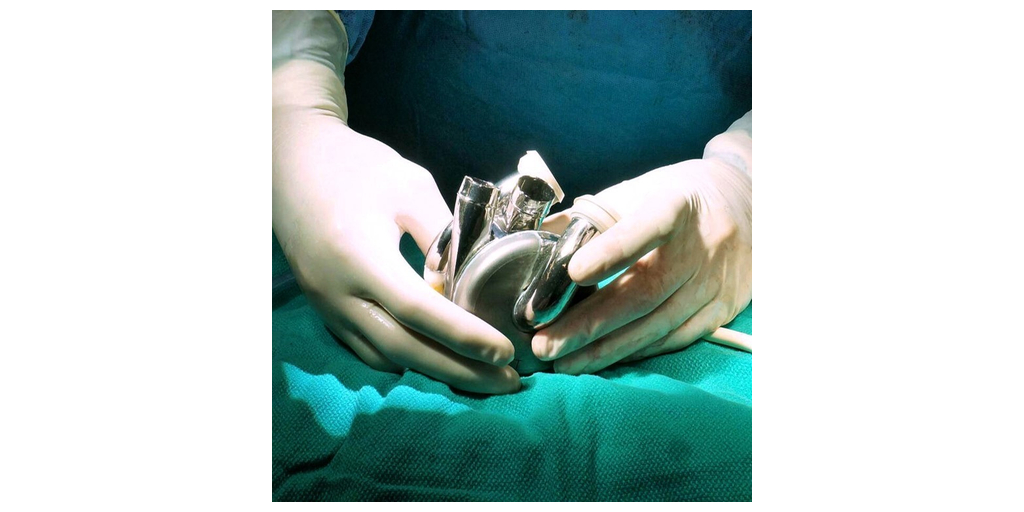
The BiVACOR Total Artificial Heart is designed as the first long-term therapy dedicated to patients with severe biventricular heart failure. It is an implantable total artificial heart based on rotary blood pump technology. Similar in size to an adult fist, it is small enough to be implanted in many women and some children yet capable of providing enough cardiac output to an adult male undergoing exercise. Using magnetic levitation (MAGLEV) technology, the same principle used in high-speed trains, the design includes left and right vanes positioned on a common rotor to form the only moving part, a magnetically suspended double-sided centrifugal impeller. Even though there are no valves or flexing ventricle chambers, the pulsatile outflow is made possible by rapidly cycling the rotational speed of the impeller. The non-contact suspension provides large blood gaps minimizing blood trauma and eliminating mechanical wear to offer a durable, reliable, and biocompatible heart replacement.
About BiVACOR®
BiVACOR® is a clinical-stage medical device company developing the BiVACOR Total Artificial Heart (TAH), which is designed to be the first long-term therapy for patients with severe biventricular heart failure. The device replaces the native heart and addresses the global unmet need of patients with end-stage heart failure by providing a next-generation life-extending solution. For more information, visit https://bivacor.com/.
Contacts
Alexander Romero-Wilson
Health+Commerce
[email protected]