Contributing to lower risk of potentially carcinogenic nitrosamine impurities in pharmaceuticals
TOKYO & NEW YORK & DÜSSELDORF, Germany--(BUSINESS WIRE)--To contribute to reducing the risk of potentially carcinogenic nitrosamine impurities in pharmaceuticals and nutritional supplements, Asahi Kasei now offers Ceolus™ microcrystalline cellulose (MCC) with nitrite levels of 0.1 μg/g (ppm) or less.
In 2018, a potentially carcinogenic nitrosamine impurity was detected in several pharmaceuticals. Since then, public awareness of the potential health hazards of nitrosamine has increased significantly worldwide. Guided by regional authorities such as the European Medicines Agency (EMA) and the U.S. Food & Drug Administration (FDA), the pharmaceutical industry has been carrying out extensive assessments and research to identify the cause of the impurities.
One risk factor for the formation of nitrosamine is nitrosation – a reaction of secondary or tertiary amines with nitrites during or after the manufacturing process of drug substances and drug products. Reducing the concentration of nitrites in raw materials for pharmaceuticals is considered to be an effective way to lower the risk of nitrosamine formation.
Minimizing nitrite level in various Ceolus™ grades
Asahi Kasei has been manufacturing Ceolus™ microcrystalline cellulose (MCC) in Nobeoka, Miyazaki, Japan since 1970. MCC is made from natural pulp, mainly used as an excipient for pharmaceuticals, nutritional supplements, and foods. Ceolus™ is primarily used as a tablet binder, an additive that provides shape and increases the volume of pharmaceutical tablets. High-performance grades of Ceolus™ in particular enable pharmaceutical and nutritional supplement manufacturers to realize challenging formulations, solve tableting issues, and produce unique and patient-friendly dosage forms.
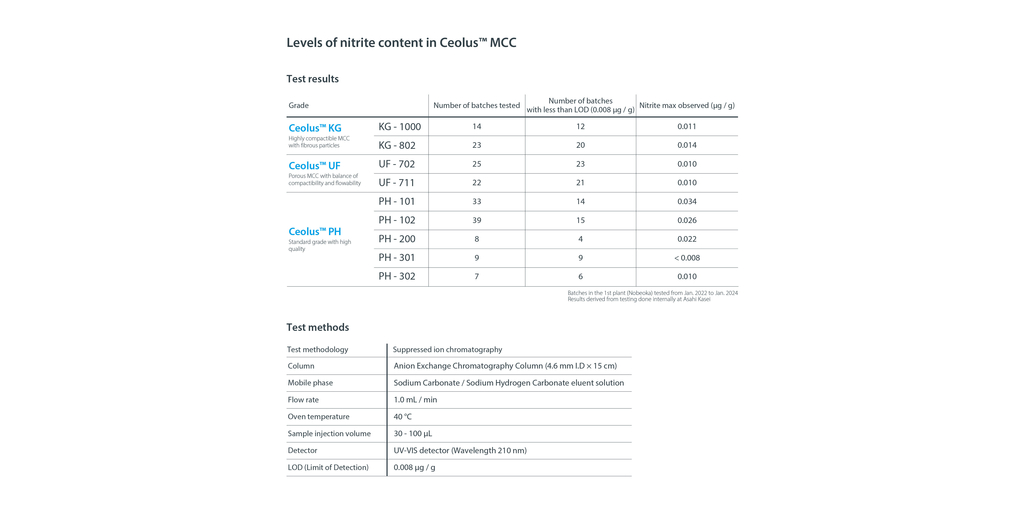
In order to reduce potential health hazards due to nitrosamine impurities, Asahi Kasei has succeeded in maintaining the nitrite concentration of Ceolus™ to 0.1 ppm or less (see image).
“Controlling the nitrite concentration in our products is an important step to ensure the provision of safer pharmaceuticals and nutritional supplements. We will continue to work on further improvements, and emphasize the importance of reducing the risk of nitrosamine impurities,” says Hideyuki Kimura, Senior General Manager of Asahi Kasei’s Healthcare Materials Division responsible for Ceolus™.
Start of second plant
In January 2024, Asahi Kasei started full commercial operation of its second manufacturing facility for Ceolus™ at its Mizushima Works in Okayama Prefecture, Japan. This not only raises supply capacity but also enhances the stability of supply through production at multiple sites.
For further details on Ceolus™ and its contribution to pharmaceutical safety, please visit our website at https://www.ceolus.com/en/mcc/.
About Asahi Kasei
The Asahi Kasei Group contributes to life and living for people around the world. Since its founding in 1922 with ammonia and cellulose fiber businesses, Asahi Kasei has consistently grown through the proactive transformation of its business portfolio to meet the evolving needs of every age. With more than 48,000 employees worldwide, the company contributes to a sustainable society by providing solutions to the world's challenges through its three business sectors of Material, Homes, and Health Care. Its Material sector, comprised of Environmental Solutions, Mobility & Industrial, and Life Innovation, includes a wide array of products from battery separators and biodegradable textiles to engineering plastics and sound solutions. For more information, visit https://www.asahi-kasei.com/.
Asahi Kasei is also dedicated to sustainability initiatives and is contributing to reaching a carbon-neutral society by 2050. To learn more, visit https://www.asahi-kasei.com/sustainability/.
Contacts
North America Contact:
Asahi Kasei America Inc.
Christian Okeefe
[email protected]
Europe Contact:
Asahi Kasei Europe GmbH
Sebastian Schmidt
[email protected]