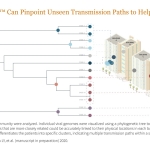
NGS assay for profiling SARS-CoV-2 genomic and subgenomic RNA in clinical specimens has compelling use cases for rapid public health response, research, and vaccine development
SINGAPORE--(BUSINESS WIRE)--Molecular diagnostics company Lucence today announced the availability of the world�s first assay kit to directly profile SARS-CoV-2 subgenomic RNA (sgRNA), a marker of active viral replication1, from clinical samples. DeepMARK� utilizes Lucence�s proprietary ultrasensitive next-generation sequencing (NGS) technology, AmpliMARK�, to concurrently detect and analyze the genome and transcriptome of SARS-CoV-2.
Pinpointing sources of unlinked SARS-CoV-2 cases supports rapid public health response. Using high-quality genetic fingerprinting, DeepMARK� can enable rapid community case tracing by identifying transmission paths, clusters, and viral contagiousness. DeepMARK��s increased sensitivity also allows for asymptomatic and recovering cases to be more thoroughly evaluated for contagiousness.
For researchers studying contagiousness, SARS-CoV-2 sgRNA is a recognized marker of active viral replication linked with contagiousness2 and offers several advantages. While viral culture is the gold standard, it is slow, expensive, and requires a biosafety level 3 (BSL3) laboratory, requirements prohibitive for the vast majority of samples. Using DeepMARK�, clinical samples can be safely and efficiently profiled using a simple workflow.
SgRNA as a marker of viral replication is also a well-recognized efficacy measurement in SARS-CoV-2 vaccine development3. Thus, comprehensive sgRNA profiling by DeepMARK� could facilitate more efficient vaccine discovery.
�Genetic fingerprinting enables quicker and deeper analysis of viral spread. This tool has enhanced our capacity to understand SARS-CoV-2 precisely and contributes to the ongoing fight against COVID-19.� said A/Prof Hsu Li Yang, Vice Dean, Global Health, Saw Swee Hock School of Public Health, National University of Singapore.
DeepMARK� is part of Lucence�s suite of SARS-CoV-2 molecular diagnostics. Lucence also makes the SAFER� Sample Kit, a saliva stabilization kit with reported 36% higher sensitivity for detecting COVID-19 compared to nasopharyngeal swabbing4.
About DeepMARK�
DeepMARK� is a next-generation sequencing (NGS) assay kit for the qualitative analysis of SARS-CoV-2 RNA. For Investigational Use Only. The performance characteristics of this product have not been established.
About Lucence
Lucence is a molecular diagnostics company that makes state-of-the-art, highly sensitive tests for disease detection and treatment selection. Headquartered in Singapore and California, Lucence�s services are delivered worldwide through an accredited central laboratory. For more information, contact enquiry@lucence.com or visit www.lucence.com/deepmark.
References
1. https://www.nature.com/articles/s41586-020-2196-x
2. https://wwwnc.cdc.gov/eid/article/26/11/20-3219_article
3. https://www.nejm.org/doi/full/10.1056/NEJMoa2024671
4. https://www.medrxiv.org/content/10.1101/2020.08.13.20173807v1
Contacts
Gabriel Yap
media@lucence.com